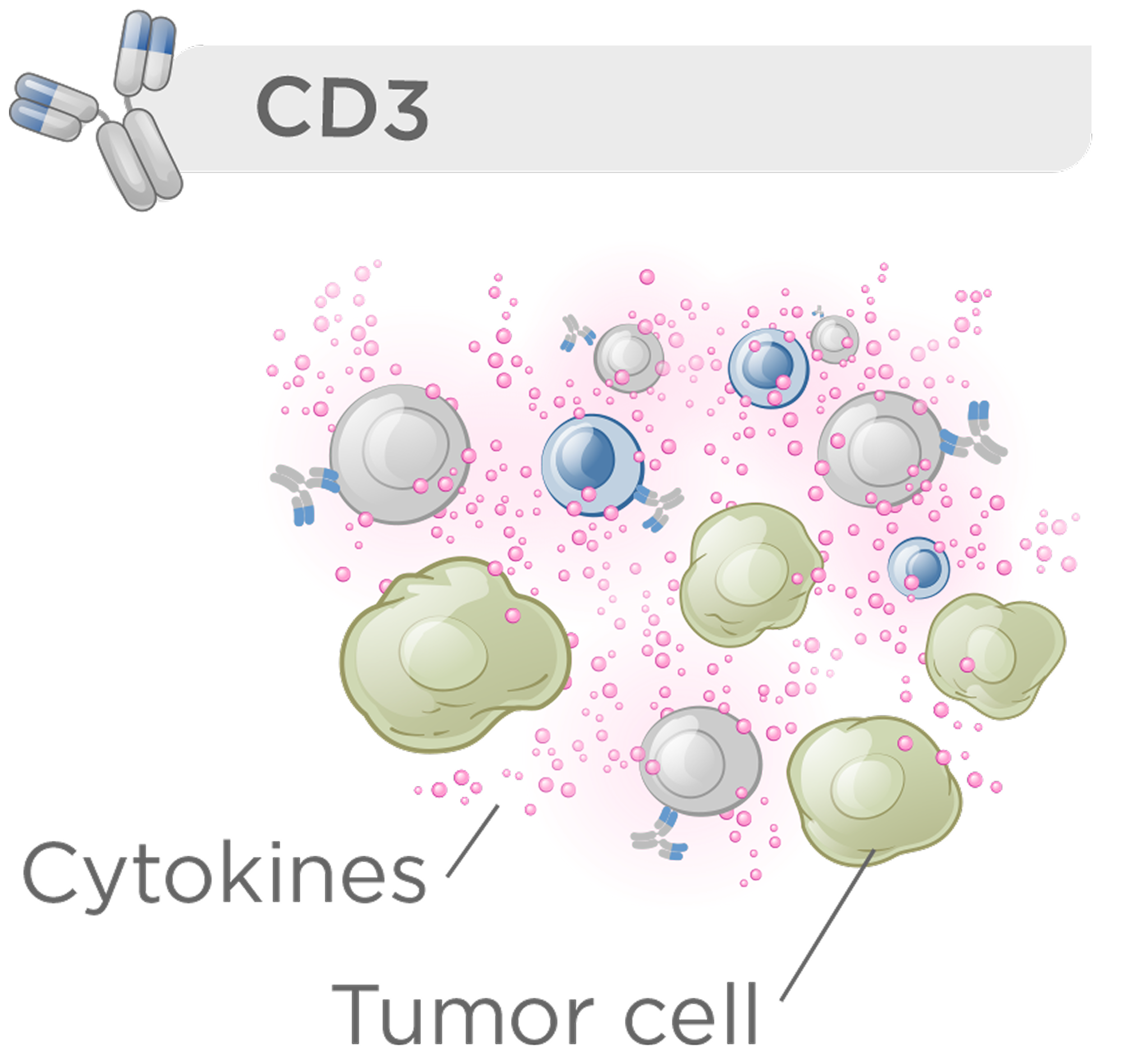
CD3 bispecific therapies that activate the majority of T cells offer clinical benefit but are constrained by a narrow therapeutic index due to immune-related toxicities such as cytokine release syndrome.
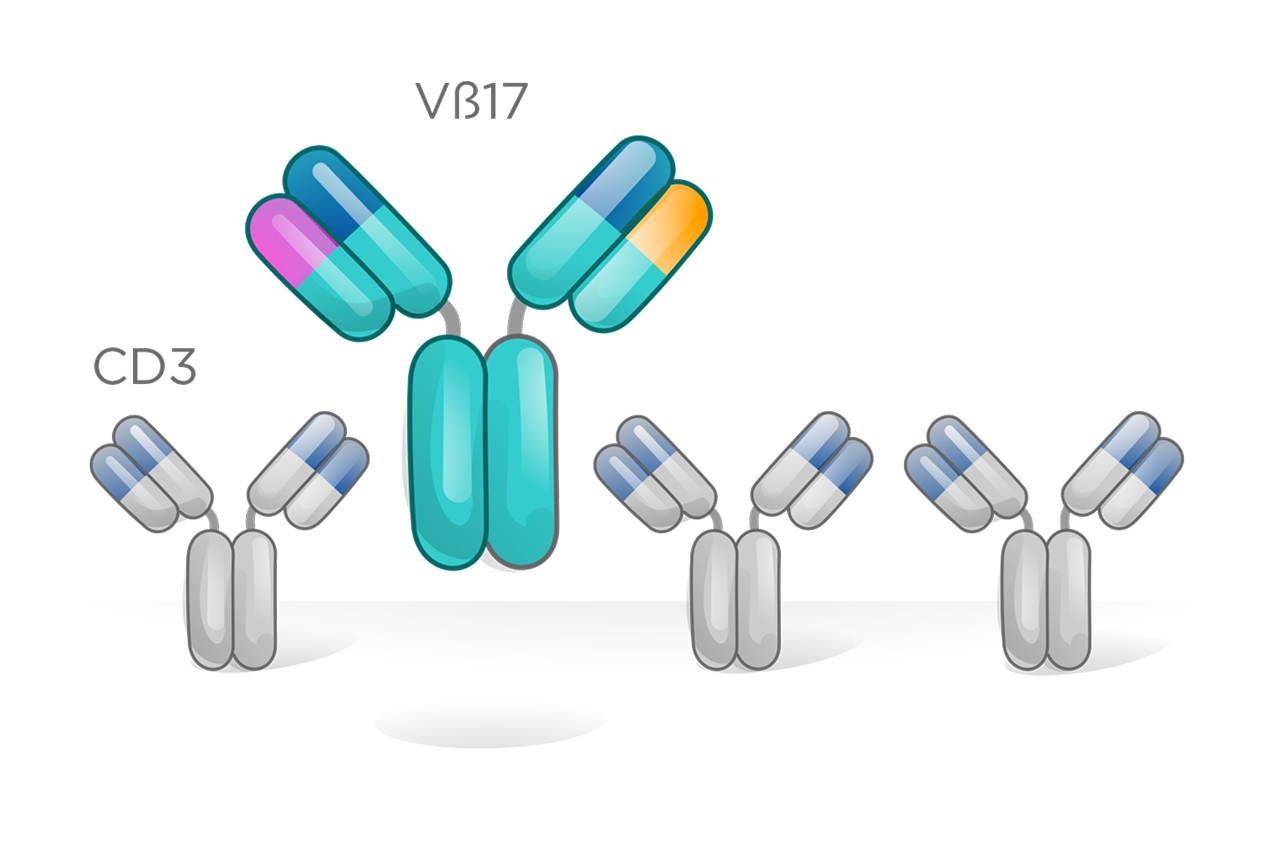

With our Vβ17 bispecific platform, we have rapidly advanced clinical candidates in both oncology and autoimmune diseases, supported by robust preclinical data demonstrating clear differentiation from conventional CD3 T cell engagers.
Our optimized protein-engineering capabilities enable fast program generation and de-risked manufacturability, accelerating progression into the clinic.
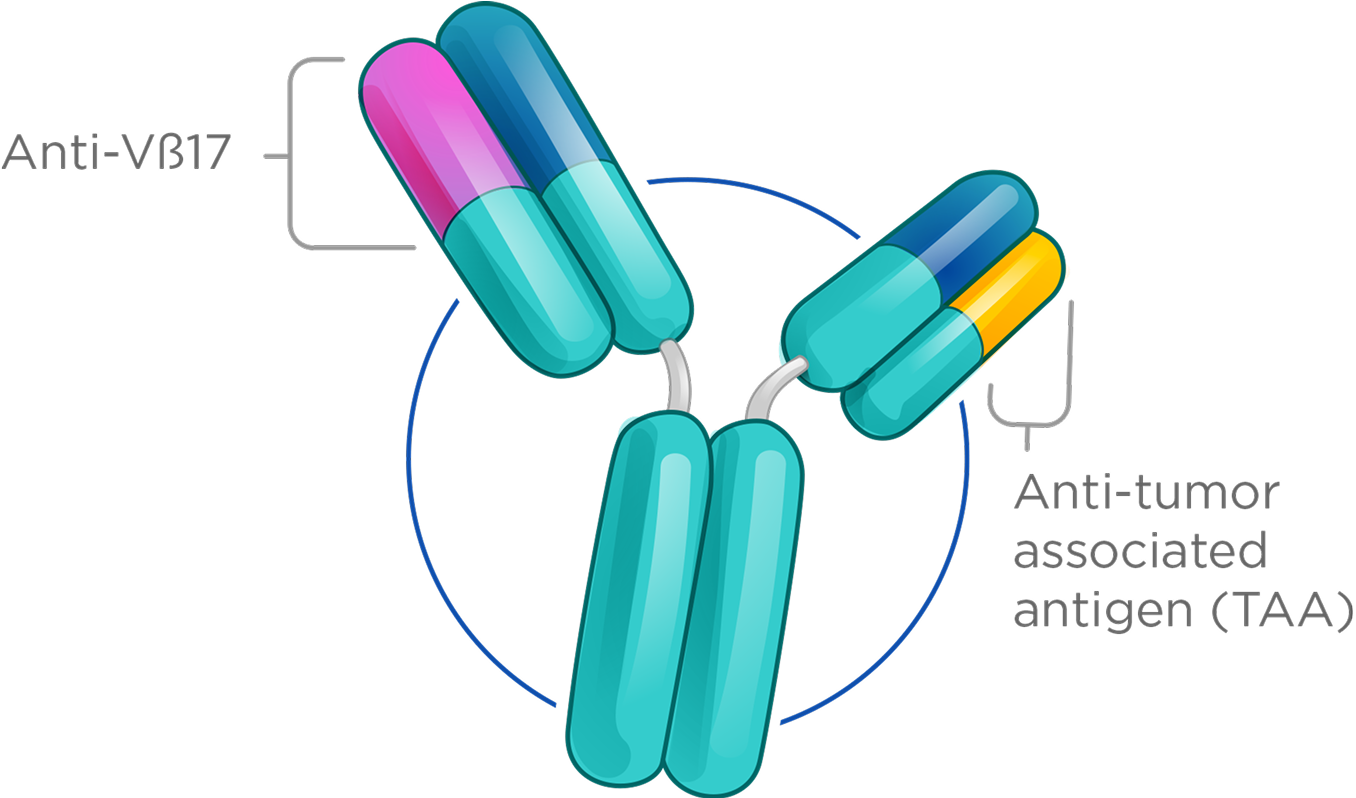
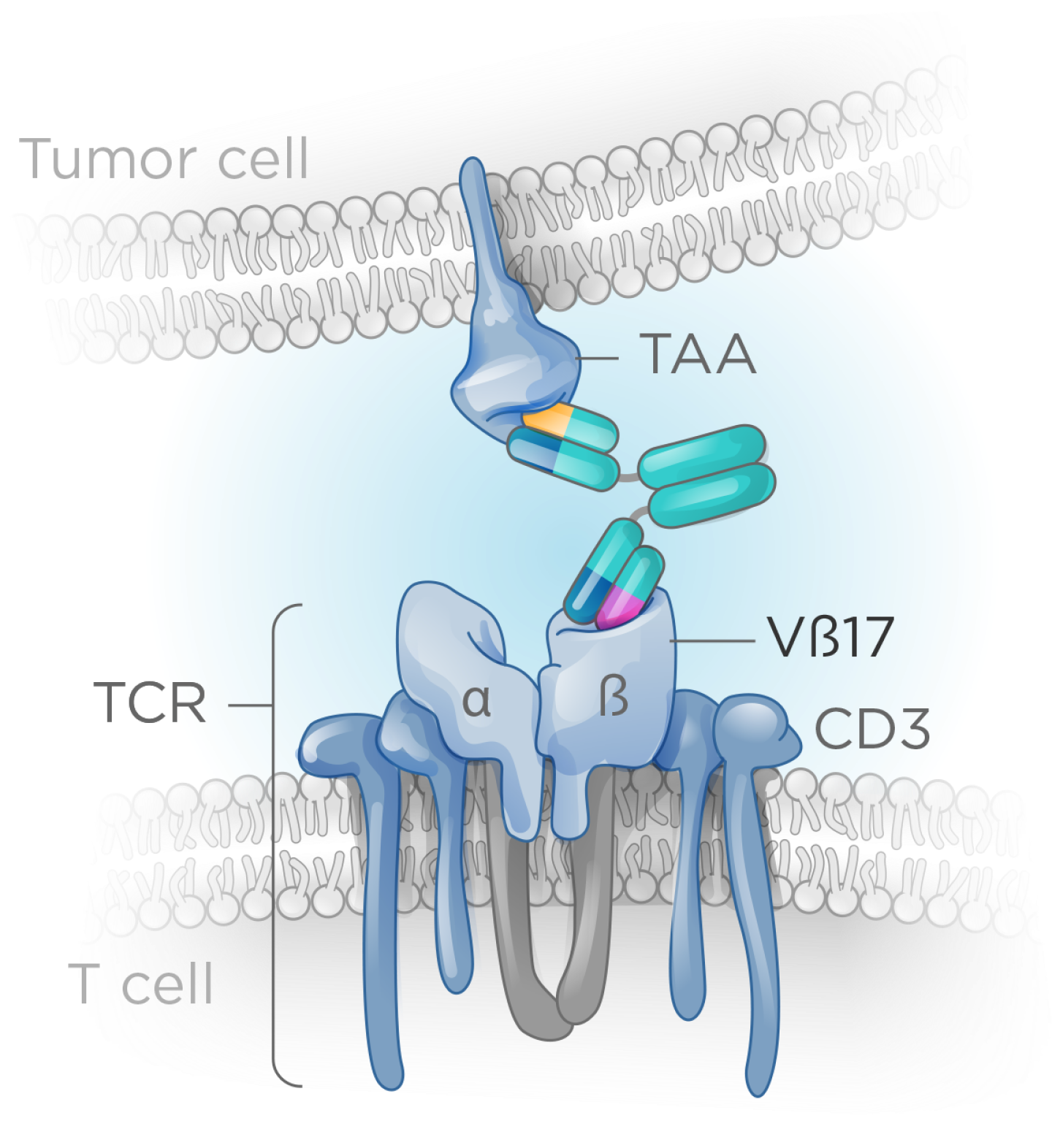
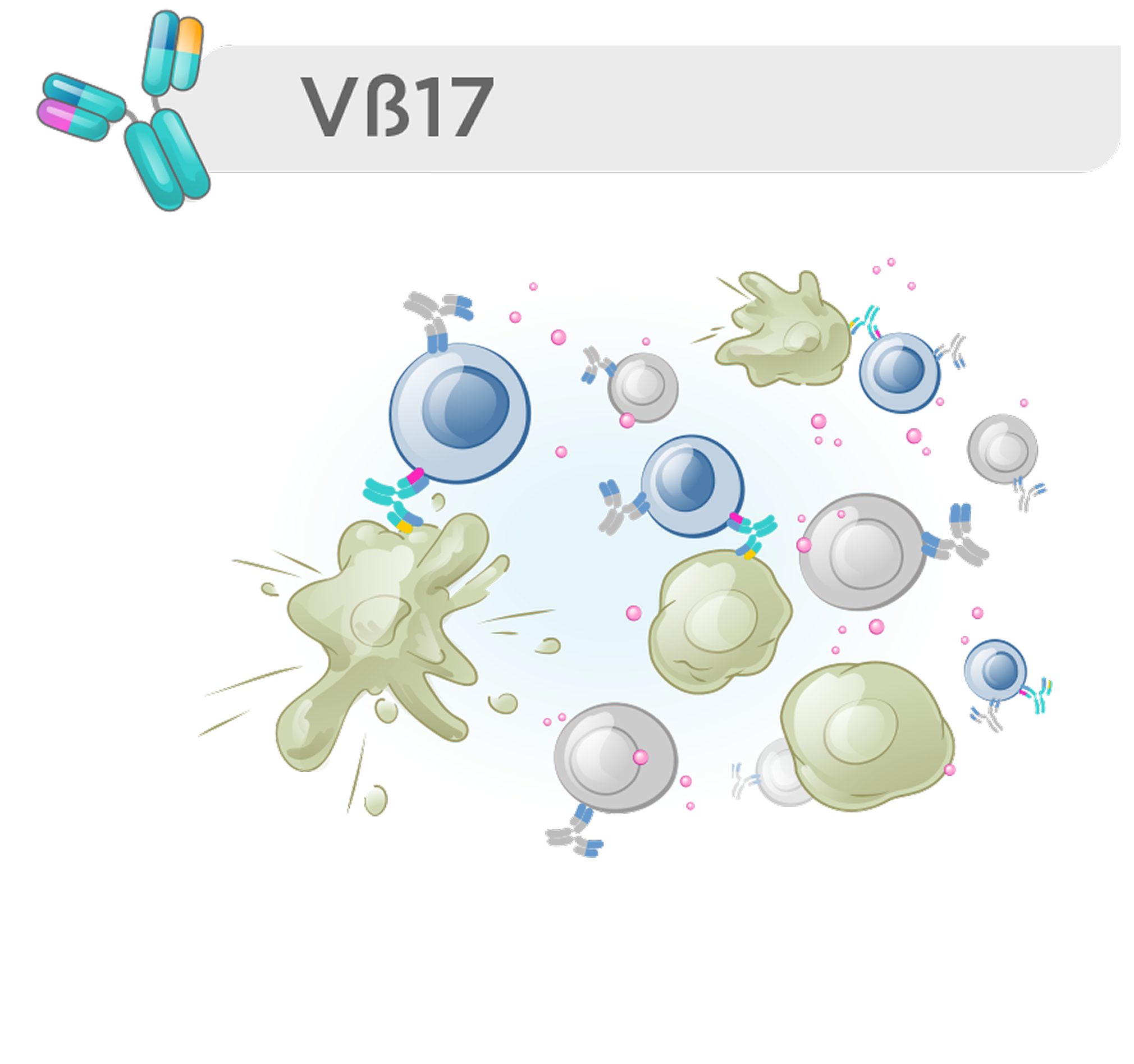
We have focused on redirecting a specific T memory subpopulation whose unique features enable a higher therapeutic index and more durable responses.
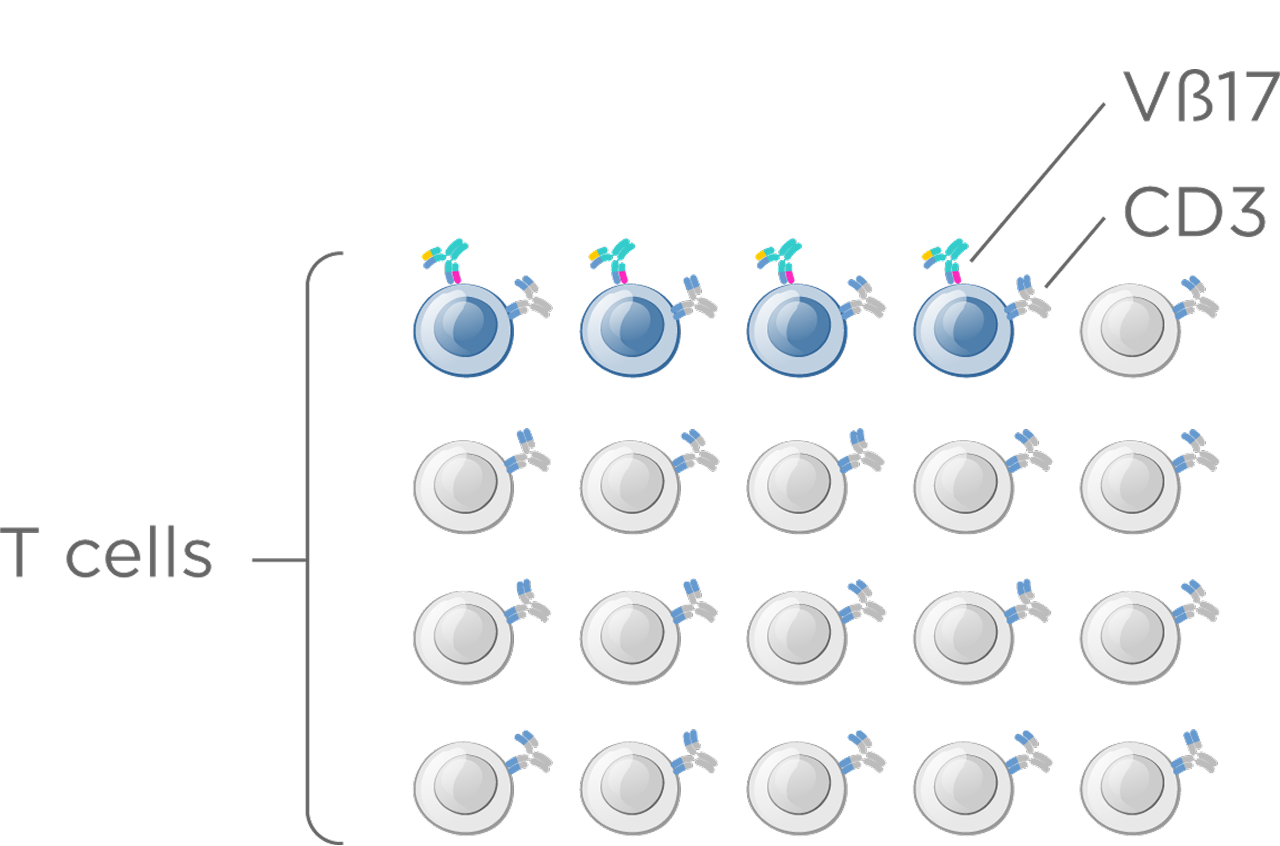
T memory cells are a subpopulation the total T cell population (~5–10%), yet they deliver robust activation and stronger, more durable cytotoxic responses than naïve T cells.
These cells can be identified by unique T cell receptor β-chains, enabling far more selective immune engagement. One such marker, Vβ17, defines a distinct T memory subset with properties that support a meaningfully improved therapeutic index—offering the potential for safer, more effective T cell–redirecting therapies.
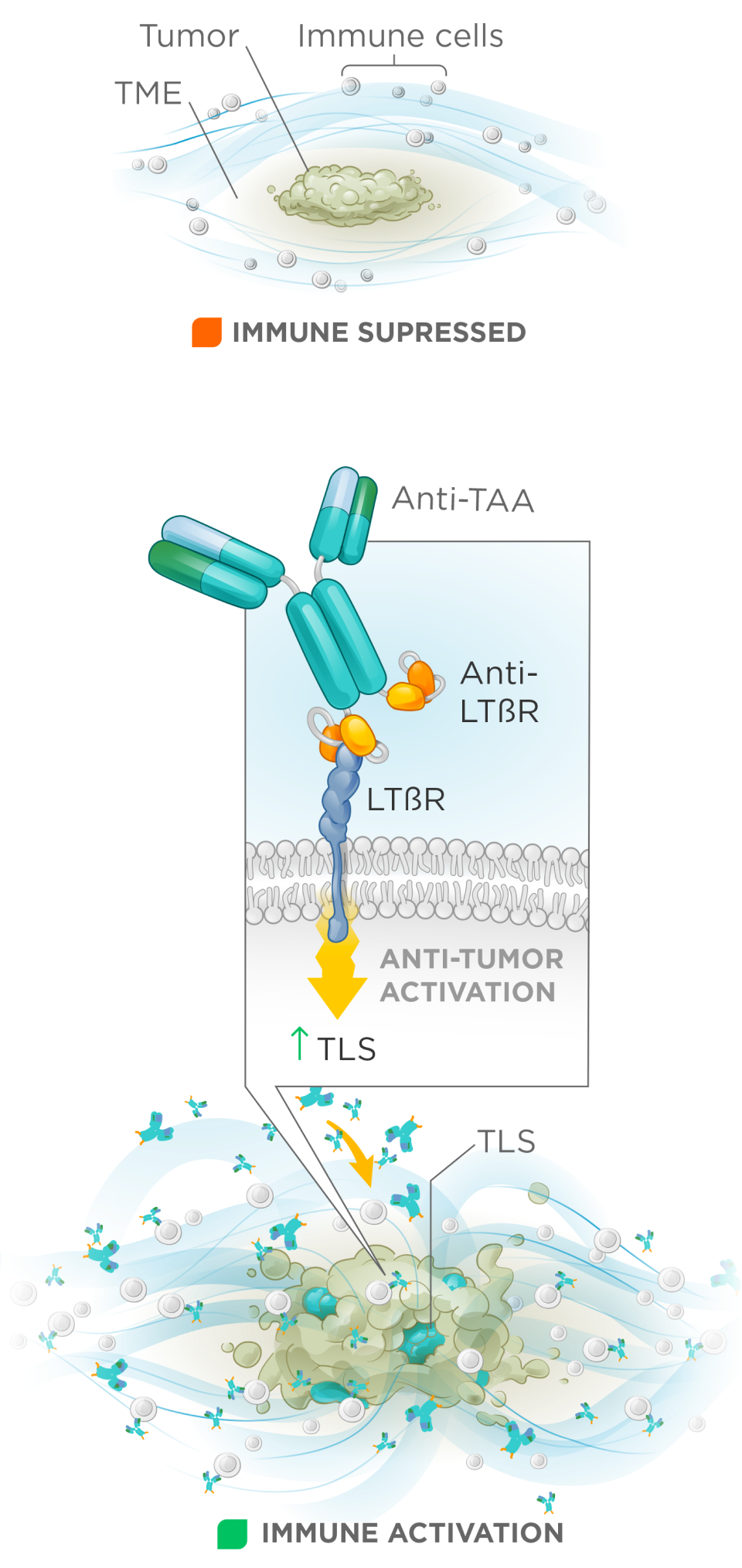
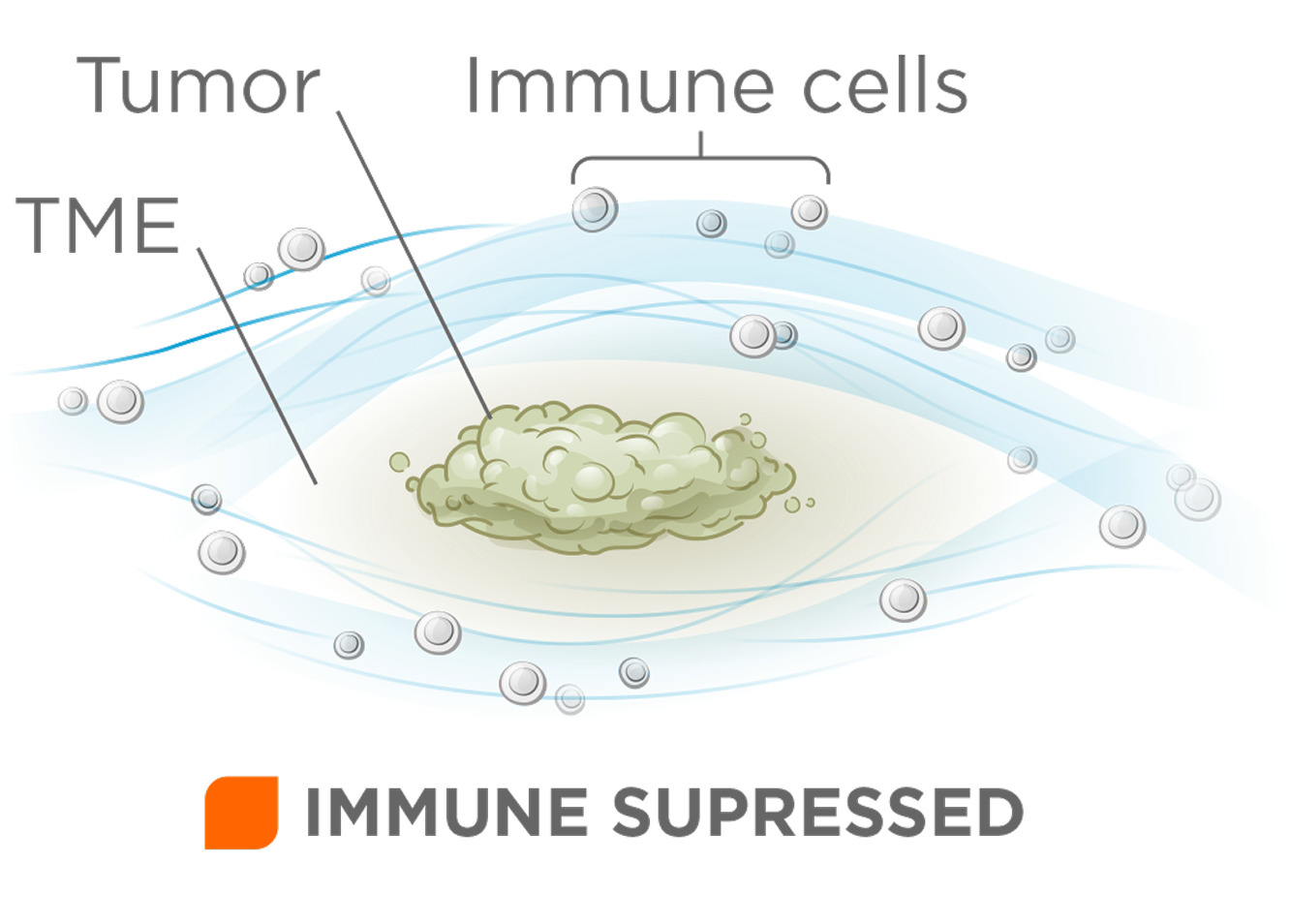
While checkpoint inhibitors have reshaped treatment for some solid tumors, the majority of patients do not respond because of tumor microenvironments (TMEs) which create immune restrictions that block immune cell trafficking and activation. Tertiary lymphoid structures (TLS) play a key role in orchestrating effective anti-tumor immunity and are strong predictors of checkpoint therapy benefit.
Formation of TLS is controlled by the TNFR family member, LTβR, enabling targeted immune recruitment, antigen presentation, and T cell activation.
We have advanced LTβR bispecifics that are highly optimized for tumor antigen dependent activation of LTβR to drive anti-tumor efficacy.
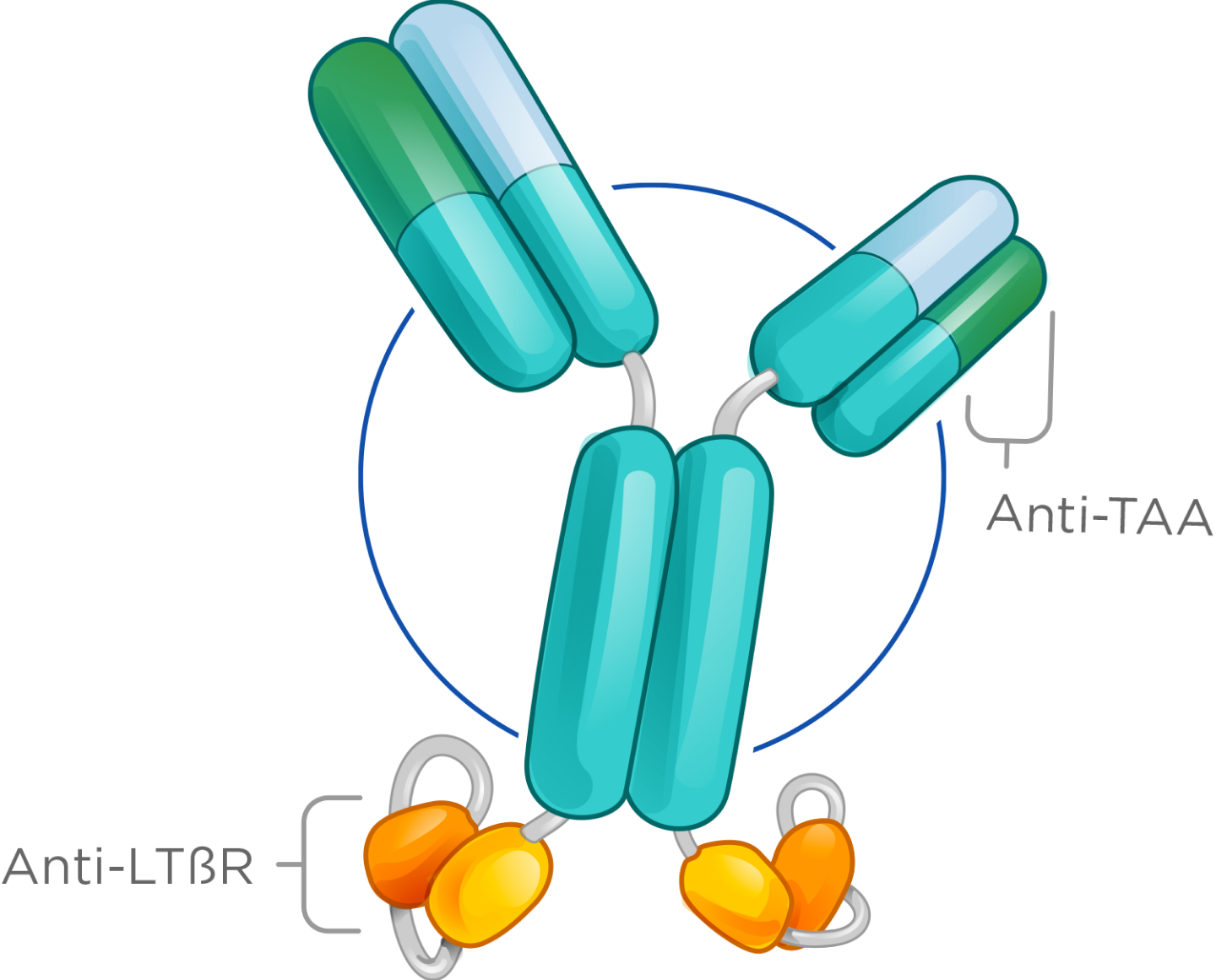
Our LTβR bispecifics show strong in vivo monotherapy activity, synergize effectively with checkpoint inhibitors, and possess favorable manufacturability, enabling clinical advancement—particularly in solid tumors that currently do not benefit from checkpoint therapy.